QBC Malaria Test
Utilizing patented QBC Capillary Tube Technology, the QBC Malaria Test is able to provide rapid malaria detection with a greater level of sensitivity than traditional Giemsa thick film methods. With all of the necessary staining agents contained within a single tube, this test is simple and efficient needing less than ten (10) minutes combined for both preparation and review.
Designed to be used with a fluorescent microscope, the QBC Malaria Test represents an innovative solution in malaria testing technology.
Literature
Brochure
A full-color brochure describing the many features and benefits of the QBC Malaria Test
English / Español / Français
Specification Sheet
A document containing all specifications of the QBC Malaria Test, including size, fluorescence specifications, environmental specifications, and more
English
Product Description List
A list of all configurations and accessories currently available for the QBC Malaria Test, including part numbers
English / Español / Français
Product Insert
This application note serves as a reference for using the ParaLens Advance with the QBC Malaria Test
English
User Guide: Sample ProcessingA full color, step-by-step guide to sample processing with the QBC Malaria Test
English
User Guide: Sample Review
A full color, step-by-step guide to sample review with the QBC Malaria Test
English
Atlas
A full color aid to guide the microscopist in recognizing the forms of parasites as they appear in the QBC method and to compare them with the conventional blood films for diagnosis, species determination, and quantiation of parasitemia
English
QBC Malaria Test Kit (MSDS)
A full color Material Safety Data Sheet for the QBC Malaria Test Kit
English
World Health Organization Policy Statement
In this official policy statement, the World Health Organization recommends the use of LED fluorescence microscopy for the detection of tuberculosis
English
Features
The QBC Malaria Test is a fluorescence microscopy-based malaria diagnostic test that speeds and simplifies malaria detection, with a combination of features and benefits unmatched by competing products:
- Increased Sensitivity – compared to thick film analysis, with proven advantages in cases of low parasitemia.
- Time Savings – Results in as low as eight (8) minutes, a fraction of the time of thick film analysis. Reduced training time for new users.
- Simple Set-Up and Review – Improved sample collection and review for users through patented QBC technology.
- Respected, Proven Technology – Boasted FDA clearance and a long history of proven success in dozens of scientific studies.
Increased Sensitivity
Studies have shown that the QBC Malaria Test is 5.5 to 7 times more sensitive than Giemsa thick films.¹ The QBC Test also offers equivalent specificity with a negative predictive value of greater than 98%.²
The sensitivity of the QBC Malaria Test is particularly notable in cases of low parasitemia, as the test allows for the detection of as little as one (1) parasite per µL of blood.³ In one study of 49 patients with low parasitemia (defined as >10 parasites per µL of blood), the QBC Test established the diagnosis earlier than thick film in 47% of cases.⁴
Time Savings
Preparation and review of the QBC Malaria Test can be performed in just eight (8) minutes, compared to times ranging from 45 minutes to 2 hours using thick film analysis.⁵
Training new users is just as rapid, as users with just five (5) days of training can provide results equivalent to an experienced microscopist using conventional methods.² In a study of 206 samples, new users averaged sensitivity of 95%, specificity of 98.4%, and correct speciation in 93.4% of cases.
Simple Set-Up and Review
The QBC Malaria Test utilizes patented technology to improve sample collection and review for users. The Test features a 50 µL blood tube that can be used to collect either capillary or venous blood samples. All necessary stains and reagents are contained within the tube itself, simplifying specimen processing.
The use of centrifugation improves review for users, concentrating parasites within specific areas of the tube for easier detection. For a discussion on the scientific principles behind the QBC Malaria Test, please consult the technology section.
Respected, Proven Technology
The QBC Malaria Test was the first malaria diagnostic test to receive FDA clearance and its technology has been proven in dozens of scientific studies. For a listing of scientific studies featuring the QBC Malaria Test, please consult the Studies section.
1. Bentio, A.; Roche, J.; Molina, R.; Amela, C.; Alavar, J. (1994): Application and Evaluation of QBC Malaria Diagnosis in a Holoendemic Area. Applied Parasitology. Vol. 35: 266-272.
2. Namsiripongpun, V.; Pansamdaent, P.; et. Al. (1990): The Acridine Orange Stained Capillary Tube (The QBC System) in Diagnosis of Malaria: A Field Trial. J. Prapokklao Hospital Clinic Education Center. Vol. 7, No. 2.
3. Ponsilapatip, J.; Namsiripongpun, V.; et. Al. (1990): Detection of Plasmodia in Acridine Orange Stained Capillary Tubes (The QBC System). Southeast Asian Journal of Tropical Medicine and Public Health. Vol. 21, No. 4.
4. Tangpukdee, N.; Dangdee, C.; Wilairatana, P.; Krudsood, S. (2009): Malaria Diagnosis. Korean Journal of Parasitology. Vol. 47, No. 2: 93-102.
5. Oloo, A.; Ondijo, S.; Genga, I.; Boriga, D.; Owaga, M.; Ngare, D.; Gathecha, E. (1994): Evaluation of the QBC Method to Detect Malaria Infections in Field Surveys. East African Medical Journal. Vol. 71, No. 5.
Technology
The QBC Malaria Test delivers its unmatched benefits through a unique, patented combination of technological advances:
- Acridine Orange Stain makes malaria parasites more easily visible through the use of fluorescence microscopy
- Centrifugation concentrates parasites in specific areas of the tube for improved detection
- Larger blood samples contain more parasites than samples used for Giemsa stains, providing benefits in cases of low parasitemia
Acridine Orange Stain
The interior of the QBC Malaria Test is coated with fluorescent acridine orange stain, which bonds with the nucleic acid of any malaria parasites present in the sample during preparation.
When viewed under blue light (~460 nm), parasites stained with acridine orange will fluoresce brightly against a dark background. This greatly increases 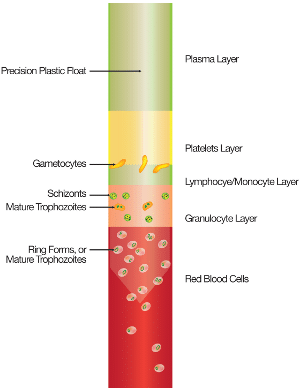 visibility, contributing to improved sensitivity.
visibility, contributing to improved sensitivity.
Centrifugation
Before review, the QBC Malaria Test is centrifuged at 14,400 xg (12,000 RPM) for five (5) minutes. During the process, components of blood separate into distinct layers based on their differing densities.
Parasites contained in the sample will also separate according to density, as seen in the illustration of a tube containing P. falciparum malaria on the right. This simplifies detection for users, as the parasites will concentrate into specific, easy-to-locate areas of the tube.
Larger Blood Samples
A typical Giemsa stain uses just 2 µL of blood. In a case of low parasitemia (~1 parasite per µL), a thick film sample will contain just two parasites, making detection a difficult and time-consuming task.
The QBC Malaria Test concentrates a 50 µL sample, meaning that, in cases of low parasitemia, approximately 50 parasites will be present. Because the sample is concentrated, parasites are easy to locate so review of this larger sample will take no additional time.
Frequently Asked Questions
At what temperature should I store the QBC Malaria Test?
The QBC Malaria Test can be stored from 16°C to 37°C (60°F to 99°F).
How long can a centrifuged QBC Malaria Test be stored prior to review?
Although it is recommended to review a QBC Malaria Test immediately after preparation, it is possible to store a centrifuged test for three (3) days at 16°C to 37°C (60°F to 99°F) or two weeks at 4°C (39°F).
What kind of microscope is required to review a QBC Malaria Test?
The QBC Malaria Test is designed to work with a fluorescence microscope. Drucker Diagnostics offers the QBC ParaLens Advance Microscope Attachment which can provide fluorescence capabilities to a standard light microscope.
What kind of centrifuge is required to prepare a QBC Malaria Test sample?
The QBC Malaria Test must be centrifuged at 14,400 xg rpm. the QBC Capillary Centrifuge was specifically designed to spin QBC Malaria Tubes, as well as other QBC blood tubes, to this specification.
How do I focus my microscope on the QBC Malaria Test?
If you’re using a ParaLens Advance lens of 60x magnification, apply 2 to 3 drops of immersion oil to the buffy coat layer of the tube. From a side perspective, visually line the lens to the buffy coat layer (the buffy coat layer will appear slightly fluorescent when aligned). Gradually lower the lens until it touches the oil. There will be a distinct wicking effect when they touch.
Look through the eyepieces and begin to focus downward. When part of the sample is in focus, adjust the Y-axis until most of the surface area is in focus. Once a sufficient percentage is in focus, refrain from adjusting the Y-axis. Instead, manually rotate the tube using the tube closure.
What type of sample is required for QBC Malaria Test testing?
Whole blood samples collected from either a capillary stick or a venous blood draw (EDTA purple top tube).
How much blood is required to test on the QBC Malaria Test?
The QBC Malaria Test requires 55 to 65 µL of blood.
How long can a venous sample be stored prior to testing in the QBC Malaria Test?
Refrigerated samples stored at 2°C to 8°C (35°F to 46°F) are stable for up to eight (8) hours.
Note – Bring the sample back to room temperature before you prepare the Malaria Test
How do I know if what I’m looking at is malaria?
Malaria has several distinctive characteristics, many of which are unique to the species and life-cycle stage. For more detailed instructions on detecting and identifying malaria, please consult our training and literature resources.
Can I quantify using the QBC Malaria Test?
The QBC Malaria Test provides an accurate and reproducible method of estimating the relative quantity of parasites using the “Plus System”:
- +(1+) = >1 parasite per QBC field
- ++(2+) = 1 – 10 parasites per QBC field
- +++(3+) = 11 – 100 parasites per QBC field
- ++++(4+) = <100 per QBC field
This system can aid in following the progress of a patient under therapy and in comparing the level of parasitemia from one patient to another.
For complete quantification, no algorithm has been uniformly accepted in scientific literature. A study conducted by Benito et al. (Application and evaluation of QBC malaria diagnosis in a holoendemic area. Feel free to visit our studies page to read more) offers a possible algorithm for use with the QBC Malaria Test.
Can I speciate using the QBC Malaria Test?
For proper speciation, many factors must be considered, including malaria morphology, location in the QBC tube, ratios between life forms, presence of multiple infections in a single RBC, morphology of gametocytes, staining characteristics, number of merozoites per schizont, and other characteristics. Reports have indicated that it is possible to speciate using the QBC Malaria Test, but it takes experience (Pornsilapatip et al., Detection of Plasmodia in Acridine Orange Stained Capillary Tube (The QBC System); Rickman et al., Rapid Diagnosis of Malaria by Acridine Orange Staining of Centrifuged Parasites. Feel free to visit our studies page to read more.
In all cases, a thin film should be used for verification.
Economics
The QBC Malaria Test can provide results in just eight (8) minutes, compared to 45 minutes to 2 hours with Giemsa Thick Films. This speed improvement allows users to increase lab throughput and make the most of their limited resources.
- Collection and preparation of sample using finger stick or venous blood draw – 2 Minutes
- Centrifugation of sample with the QBC Capillary Centrifuge – 5 Minutes
- Analysis of sample with ParaLens Advance or other fluorescence microscope – 1 Minute
- Total Time – 8 Minutes
Additionally, paratechnicians with only five (5) days of training on the QBC Malaria Test can achieve results comparable to technicians with months of experience using thick and thin films. That means less time spent in the classroom and more time spent providing tests and other services for patients.
Service
For all service inquiries, please contact the Drucker Diagnostics Technical Service Department at +1-866-265-1486 (US only) or support@druckerdiagnostics.com. Our Technical Support Representatives are available Monday – Friday, 8:30 am to 5:00 pm (EST).
Training
There are several resources currently available for use in preparing and reviewing the QBC Malaria Test. The Malaria Test Product Insert contains step-by-step instructions on using the QBC Malaria Test. Two Malaria Test User Guides, featuring full-color illustrations and photos, are available to help users of the test. The User Guide Sample Processing walks users step-by-step through the preparation of samples with the QBC Test, while the User Guide: Sample Review is designed to provide assistance to users during review. Finally, our ParaLens Advance/QBC Malaria Test Application Note contains many tips for using the QBC Malaria Test with the ParaLens Advance system.
Studies
The following scientific studies on the QBC Malaria Test technology are available from external sources, such as PubMed.gov (https://www.ncbi.nlm.nih.gov/pubmed/)
Diagnosis of Hematoparasites using Quantitative Buffy Coat Analysis (QBC)
Spielman A, Perrone JB, Teklehaimanot A, Balcha F, Wardlaw SC, & Levine RA, 1988: Malaria diagnosis by direct observation of centrifuged samples of blood. Am J Trop Med Hyg., 39 (4), 337-42.
View Link
Levine RA, Wardlaw SC, & Patton CL, 1989: Detection of haematoparasites using quantitative buffy coat analysis tubes. Parasitol Today, 5 (4), 132-4.
View Link
Malaria Studies (General)
Jain M & Kaur M, 2005: Comparative study of microscopic detection methods and haematological changes in malaria. Indian J Pathol Microbiol, 48 (4), 464-7.
View Link
Malik S, Khan S, Das A, & Samantaray JC, 2004: Plasmodium lactate dehydrogenase assay to detect malaria parasites. Natl Med J India, 17 (5), 237-9.
View Link
Nandwani S, Mathur M, and Rawat S, 2003: Evaluation of the direct acridine orange staining method and QBC test for diagnosis of malaria in Delhi, India. J Commun Dis., 35 (4), 279-82
View Link
Barman D, Mirdha BR, Samantray JC, Kironde F, Kabra SK, and Guleria R, 2003: Evaluation of quantitative buffy coat (QBC) assay and polymerase chain reaction (PCR) for diagnosis of malaria. J Commun Dis., 35 (3), 170-81.
View Link
Yavo W, Ackra KN, Menan El, Barro-Kiki PC, Kassi RR, Adjetey TA, Bamba, and Kone M, 2002: Comparative study of four malaria diagnostic techniques used in Ivory Coast > French < Bull Soc Pathol Exot., 95 (4), 238-40.
View Link
Krishna BV and Deshpande AR, 2003: Comparison between conventional and QBC methods for diagnosis of malaria. Indian J Pathol Microbiol., 46 (3), 517-20.
View Link
Pinto M JW, Rodrigues SR, Desouza R, and Verenkar MP, 2001: Usefulness of quantitative buffy coat blood parasite detection system in diagnosis of malaria. Indian Journal of Medical Microbiology, 19 (4), 219-221.
View Link
Singh H, Tyagi PK, and Sharma SK, 2001: Quantitative buffy coat versus conventional microscopy. J Assoc Physicians India., 49, 945-6.
View Link
Hovette P, Aubron C, Perrier-Gros-Claude JD, Schieman R, N’Dir MC, and Camara P, 2001: Value of Quantitative Buffy Coat (QBC) in borreliasis-malaria co-infection. Med Trop (Mars)., 61 (2), 196-7.
View Link
Thakor HG, 2000: Laboratory diagnosis of malaria. J Indian Med Assoc., 98 (10), 623-7.
View Link
Biswas R, Sengupta G, and Mundle M, 1999: A controlled study on haemograms of malaria patients in Calcutta. Indian J Malariol, 36 (1-2), 42-8.
View Link
Mirdha BR, Samantray JC, Burman D, Mishra B, and Ghimire P, 1999: Quantitative buffy coat: a special adjunct for diagnosis of malaria. J Commun Dis., 31 (1), 19-22.
View Link
el Serougi AO and Amin AM, 1998: The quantitative buffy coat capillary tubes versus thin and thick blood films in the diagnosis of malaria in Saudi Arabia. J Egypt Soc Parasitol., 28 (1), 17-22.
View Link
Damodar SU, 1996: Evaluation of acridine-orange staining of centrifuged parasites in malarial infection. Indian J Med Sci., 50 (7), 228-30.
View Link
Wang X, Zhu S, Liu Q, Hu A, Zan Z, Yu Q, and Yin Q, 1996: Field evaluation of the QBC technique for rapid diagnosis of vivax malaria. Bull World Health Organ., 74 (6), 599-603
View Link
Bawden M, Malone J, and Slaten D, 1994: QBC malaria diagnosis: easily learned and effectively applied in a temporary military field laboratory. Trans R Soc Trop Med Hyg., 88 (3), 302
View Link
Long GW, Jones TR, Rickman LS, Fries L, Egan J, Wellde B, and Hoffman SL, 1994: Acridine orange diagnosis of Plasmodium falciparum: evaluation after experimental infection. Am J Trop Med Hyg., 51 (5), 613-6
View Link
Pornsilapatip J, Namsiripongpun V, Wile H, Hanvanich M, Chutivongse S, 1990: Detection of Plasmodia in acridine orange stained capillary tubes (the QBC system). Southeast Asian J Trop Med Public Health, 2 (4), 534-40.
View Link
Benito A, Roche J, Molina R, Amela C, and Alvar J., 1994: Application and evaluation of QBC malaria diagnosis in a holoendemic area. Appl Parasitol., 35 (4), 266-72.
View Link
Rickman LS, Long GW, Oberst R, Cabanban A, Sangalang R, Smith JI, Chulay JD, and Hoffman SL, 1989: Rapid diagnosis of malaria by acridine orange staining of centrifuged parasites. Lancet, 1 (8629), 68-71.
View Link
Parija SC, Dhodapkar R, Elangovan S, Chaya DR: A comparative study of blood smear, QBC and antigen detection for diagnosis of malaria. Indian J Pathol Microbiol. 2009 Apr-Jun; 52 (5), 200-2.
View Link
Alunni-Perret V, Vandenbos F, Kechkekian A, Marty P, Legros F, Michiels JF, Cardot-Leccia N, Fortineau N, Durant J, Quatrehomme G.: Fatal cerebral malaria diagnosed after death in French patient. Am J Forensic Med Pathol. 2010 (Sep); 31 (3): 269-72
View Link
J Gallagher, 2010 Screening for Malaria: the QBC technique
View PDF
Testimonials
QBC systems have been in use globally for over 25 years with active installations on every continent, including Antarctica. There are over 15,000 active hematology systems worldwide and at least 1,500 fluorescent microscopy systems using our Malaria, Parasitology, and AFB kits. If you would like references for any of our innovative solutions from users near you, please contact us.